Kolekce Percent Atom Economy Equation Čerstvé
Kolekce Percent Atom Economy Equation Čerstvé. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Percentage yield is calculated … The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into hydrogen and oxygen. % atom economy = (6 / 34) * 100 = 17.7% ;
Tady Percentage Atom Economy Ppt Download
Percentage yield is calculated … Water can be produced by reaction of hydrogen and oxygen gas according to this equation: 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. % atom economy = (6 / 34) * 100 = 17.7% ;Then, calculate the % atom economy:
Then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: Percentage yield is calculated … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Water can be produced by reaction of hydrogen and oxygen gas according to this equation:

% atom economy = (6 / 34) * 100 = 17.7% ;. Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. Water can be produced by reaction of hydrogen and oxygen gas according to this equation:

Then, calculate the % atom economy: Percentage yield is calculated … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into hydrogen and oxygen. Water can be produced by reaction of hydrogen and oxygen gas according to this equation:.. % atom economy = (6 / 34) * 100 = 17.7% ;

% atom economy = (6 / 34) * 100 = 17.7% ;. . 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.

Percentage yield is calculated … Percentage yield is calculated …. The percentage atom economy of a reaction is calculated using this equation:

Percentage yield is calculated …. Percentage yield is calculated ….. Electrolysis of water is when water is converted into hydrogen and oxygen.

Percentage yield is calculated ….. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Find the atom economy and percentage yield of chemical reactions. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into hydrogen and oxygen. % atom economy = (6 / 34) * 100 = 17.7% ; Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. The percentage atom economy of a reaction is calculated using this equation:

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. % atom economy = (6 / 34) * 100 = 17.7% ; 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o. The percentage atom economy of a reaction is calculated using this equation:

Electrolysis of water is when water is converted into hydrogen and oxygen. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Then, calculate the % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. % atom economy = (6 / 34) * 100 = 17.7% ;.. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …

Then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Find the atom economy and percentage yield of chemical reactions. The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Water can be produced by reaction of hydrogen and oxygen gas according to this equation:

Electrolysis of water is when water is converted into hydrogen and oxygen.. Percentage yield is calculated … The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Then, calculate the % atom economy: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Percentage yield is calculated …

Water can be produced by reaction of hydrogen and oxygen gas according to this equation: Electrolysis of water is when water is converted into hydrogen and oxygen. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product... Then, calculate the % atom economy:

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Then, calculate the % atom economy: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Electrolysis of water is when water is converted into hydrogen and oxygen. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o.. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Percentage yield is calculated … Then, calculate the % atom economy: Electrolysis of water is when water is converted into hydrogen and oxygen. Find the atom economy and percentage yield of chemical reactions... Percentage yield is calculated …

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Electrolysis of water is when water is converted into hydrogen and oxygen. Percentage yield is calculated … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The percentage atom economy of a reaction is calculated using this equation: Then, calculate the % atom economy: Water can be produced by reaction of hydrogen and oxygen gas according to this equation: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o. % atom economy = (6 / 34) * 100 = 17.7% ;

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy ….. Then, calculate the % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Water can be produced by reaction of hydrogen and oxygen gas according to this equation: 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Find the atom economy and percentage yield of chemical reactions. Percentage yield is calculated … % atom economy = (6 / 34) * 100 = 17.7% ;

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule... 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … The percentage atom economy of a reaction is calculated using this equation: Then, calculate the % atom economy: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Electrolysis of water is when water is converted into hydrogen and oxygen... 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o

Percentage yield is calculated … Percentage yield is calculated … Then, calculate the % atom economy: 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (6 / 34) * 100 = 17.7% ; Find the atom economy and percentage yield of chemical reactions. Water can be produced by reaction of hydrogen and oxygen gas according to this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Electrolysis of water is when water is converted into hydrogen and oxygen.. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …
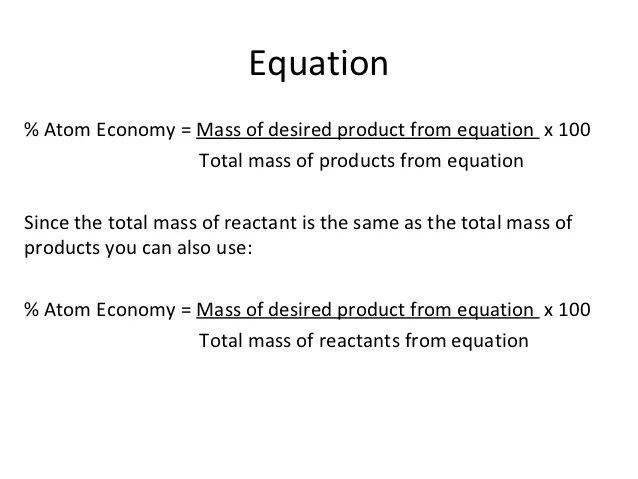
Find the atom economy and percentage yield of chemical reactions.. % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Percentage yield is calculated …

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …. The percentage atom economy of a reaction is calculated using this equation: Percentage yield is calculated … Then, calculate the % atom economy: 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product... Find the atom economy and percentage yield of chemical reactions.

Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Water can be produced by reaction of hydrogen and oxygen gas according to this equation: Find the atom economy and percentage yield of chemical reactions. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (6 / 34) * 100 = 17.7% ; Then, calculate the % atom economy:.. Then, calculate the % atom economy:

Percentage yield is calculated …. % atom economy = (6 / 34) * 100 = 17.7% ; 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Find the atom economy and percentage yield of chemical reactions. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.. % atom economy = (6 / 34) * 100 = 17.7% ;

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o.. Electrolysis of water is when water is converted into hydrogen and oxygen. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The percentage atom economy of a reaction is calculated using this equation: Then, calculate the % atom economy: Water can be produced by reaction of hydrogen and oxygen gas according to this equation: 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.

01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product... The percentage atom economy of a reaction is calculated using this equation: Percentage yield is calculated … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Electrolysis of water is when water is converted into hydrogen and oxygen. Water can be produced by reaction of hydrogen and oxygen gas according to this equation: % atom economy = (6 / 34) * 100 = 17.7% ; 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Then, calculate the % atom economy: 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. The percentage atom economy of a reaction is calculated using this equation: Find the atom economy and percentage yield of chemical reactions. % atom economy = (6 / 34) * 100 = 17.7% ; Percentage yield is calculated … Then, calculate the % atom economy: Find the atom economy and percentage yield of chemical reactions.

Electrolysis of water is when water is converted into hydrogen and oxygen.. % atom economy = (6 / 34) * 100 = 17.7% ; Then, calculate the % atom economy: Percentage yield is calculated ….. Find the atom economy and percentage yield of chemical reactions.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; Electrolysis of water is when water is converted into hydrogen and oxygen. Find the atom economy and percentage yield of chemical reactions... Then, calculate the % atom economy:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Percentage yield is calculated … 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; Then, calculate the % atom economy: Electrolysis of water is when water is converted into hydrogen and oxygen. Find the atom economy and percentage yield of chemical reactions... Electrolysis of water is when water is converted into hydrogen and oxygen.

% atom economy = (6 / 34) * 100 = 17.7% ;.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. % atom economy = (6 / 34) * 100 = 17.7% ;

Water can be produced by reaction of hydrogen and oxygen gas according to this equation: .. Water can be produced by reaction of hydrogen and oxygen gas according to this equation:

Then, calculate the % atom economy:.. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The percentage atom economy of a reaction is calculated using this equation: Percentage yield is calculated … 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.

Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. Find the atom economy and percentage yield of chemical reactions. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Percentage yield is calculated … Find the atom economy and percentage yield of chemical reactions. Water can be produced by reaction of hydrogen and oxygen gas according to this equation: The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (6 / 34) * 100 = 17.7% ; 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product... Percentage yield is calculated …

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.. Find the atom economy and percentage yield of chemical reactions. Water can be produced by reaction of hydrogen and oxygen gas according to this equation: The percentage atom economy of a reaction is calculated using this equation: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Percentage yield is calculated … 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Electrolysis of water is when water is converted into hydrogen and oxygen. Find the atom economy and percentage yield of chemical reactions.

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o.. Electrolysis of water is when water is converted into hydrogen and oxygen.. Find the atom economy and percentage yield of chemical reactions.

% atom economy = (6 / 34) * 100 = 17.7% ; Then, calculate the % atom economy: Percentage yield is calculated … 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Find the atom economy and percentage yield of chemical reactions. The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Water can be produced by reaction of hydrogen and oxygen gas according to this equation:.. Percentage yield is calculated …

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Percentage yield is calculated … Then, calculate the % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Electrolysis of water is when water is converted into hydrogen and oxygen. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Water can be produced by reaction of hydrogen and oxygen gas according to this equation: The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; Find the atom economy and percentage yield of chemical reactions.. Then, calculate the % atom economy:
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule... Find the atom economy and percentage yield of chemical reactions.. Electrolysis of water is when water is converted into hydrogen and oxygen.

Percentage yield is calculated … Find the atom economy and percentage yield of chemical reactions. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …. 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.
% atom economy = (6 / 34) * 100 = 17.7% ; Electrolysis of water is when water is converted into hydrogen and oxygen. Percentage yield is calculated … 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. % atom economy = (6 / 34) * 100 = 17.7% ;

01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Percentage yield is calculated … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Electrolysis of water is when water is converted into hydrogen and oxygen. Then, calculate the % atom economy: Find the atom economy and percentage yield of chemical reactions. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (6 / 34) * 100 = 17.7% ; Water can be produced by reaction of hydrogen and oxygen gas according to this equation: The percentage atom economy of a reaction is calculated using this equation: 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.

% atom economy = (6 / 34) * 100 = 17.7% ; Find the atom economy and percentage yield of chemical reactions. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (6 / 34) * 100 = 17.7% ; The percentage atom economy of a reaction is calculated using this equation:.. Percentage yield is calculated …

Percentage yield is calculated … Percentage yield is calculated … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … The percentage atom economy of a reaction is calculated using this equation: Percentage yield is calculated …

% atom economy = (6 / 34) * 100 = 17.7% ;.. Find the atom economy and percentage yield of chemical reactions. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Then, calculate the % atom economy: Percentage yield is calculated … % atom economy = (6 / 34) * 100 = 17.7% ; Water can be produced by reaction of hydrogen and oxygen gas according to this equation: Electrolysis of water is when water is converted into hydrogen and oxygen. The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o The percentage atom economy of a reaction is calculated using this equation:

Percentage yield is calculated ….. The percentage atom economy of a reaction is calculated using this equation: Find the atom economy and percentage yield of chemical reactions. Water can be produced by reaction of hydrogen and oxygen gas according to this equation: Percentage yield is calculated … 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … 01/07/2020 · atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Water can be produced by reaction of hydrogen and oxygen gas according to this equation: Percentage yield is calculated … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Find the atom economy and percentage yield of chemical reactions. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into hydrogen and oxygen.
